Across the ever-evolving pharmaceutical market, CMOs and small pharma companies are under immense pressure to deliver a high quality product quickly and economically while still achieving complex regulatory requirements. These operational issues — from inefficiencies in the production process to disruptions in the supply chain — can severely limit their capacity to compete and expand. These obstacles are strategic and individualistic and can be solved by streamlining processes, improving quality, building capacity, and training employees.
Streamlining Inefficient Production Processes
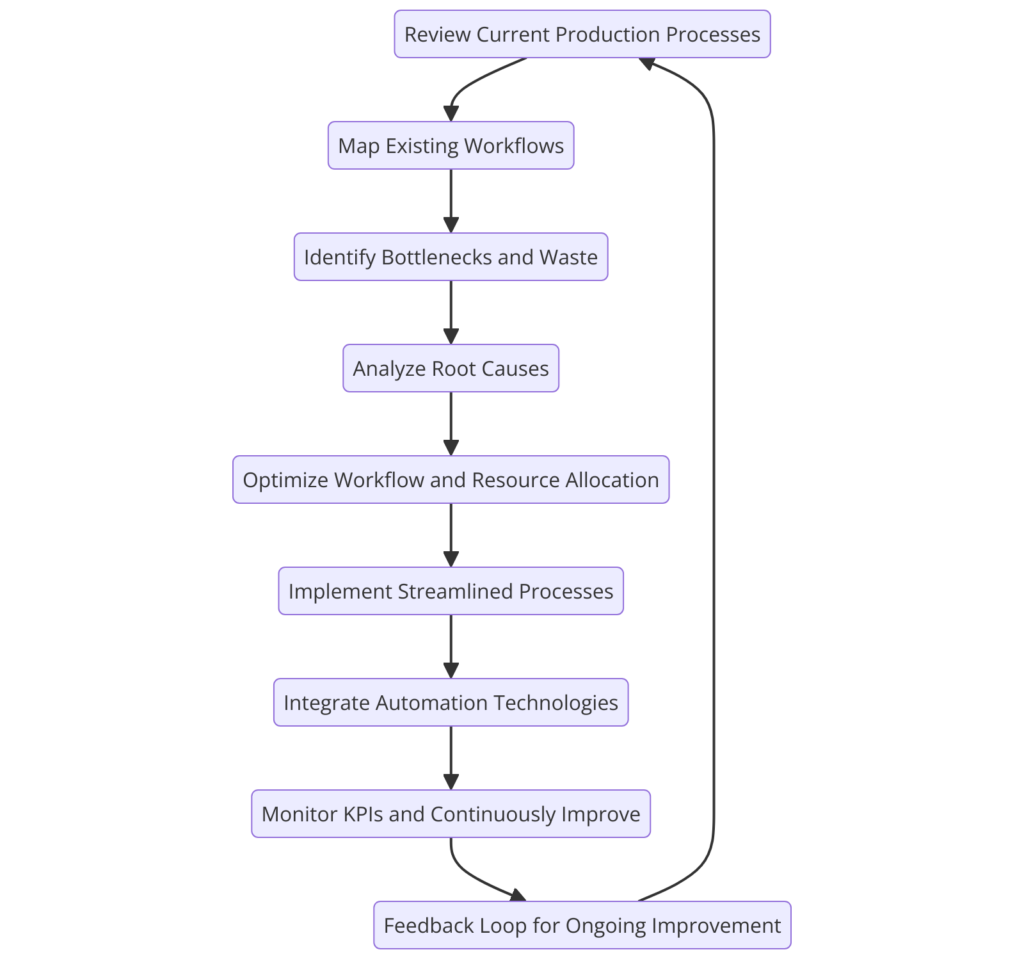
Production efficiencies are one of the biggest operational obstacles for CMOs and small pharma businesses. “Old systems, inefficiencies, manual labor, and poorly coordinated operations create bottlenecks, production delays, and higher costs. These inefficiencies decrease throughput and burden resources, thereby impairing production schedules and capacity growth.
To mitigate this, companies should be able to map processes more effectively and analyze them in a way that is consistent. By conducting a detailed review of the existing processes, organizations can reduce waste, eliminate unnecessary work and eliminate bottlenecks. By re-engineering workflows, businesses can make the best use of resources and boost overall productivity. High-level process visualizations and data-based decision support will further optimize the process to reduce production times and increase profitability.
Eliminating Waste and Boosting Efficiency in Operations
Abundant waste and poor use of resources are major drivers of higher production costs. The majority of pharmaceutical companies are afflicted by excess production, stock and non-revenue activities, which depress profits. Lean manufacturing concepts (specifically Lean Six Sigma), provide a systematic way to overcome these inefficiencies.
Through the use of the DMAIC (Define, Measure, Analyze, Improve, Control) approach, businesses can proactively identify waste and inefficiency in their business. This strategy allows businesses to deconstruct processes, identify inefficiencies at the underlying level, and take actions to improve on them. Methods like 5S (Sort, Set in Order, Shine, Standardize, Sustain) make workspaces more productive, which eliminates tons of waste and speeds up workflows. Regular use of these principles saves costs and improves productivity and product quality.
Increasing Production to Fulfill Needs
Low manufacturing capacity is one of the most common problems that keep CMOs and small pharma companies from adapting to market demand. This limitation can lead to opportunities being missed, product releases being held up, and failure to fill large orders. The key to breaking through this wall lies in the proper capacity management and use of resources.
Optimizing production capacity means detailed analyses of equipment usage, staffing, and production planning. Finding unproductive resources and redirecting them to the most urgent tasks can greatly increase productivity. Additionally, cross-training workers and accommodating flexible hours can enhance labor productivity. By implementing scalable machines and designing efficient facilities, businesses can seamlessly scale up as demand increases.
Ensuring Consistent Product Quality
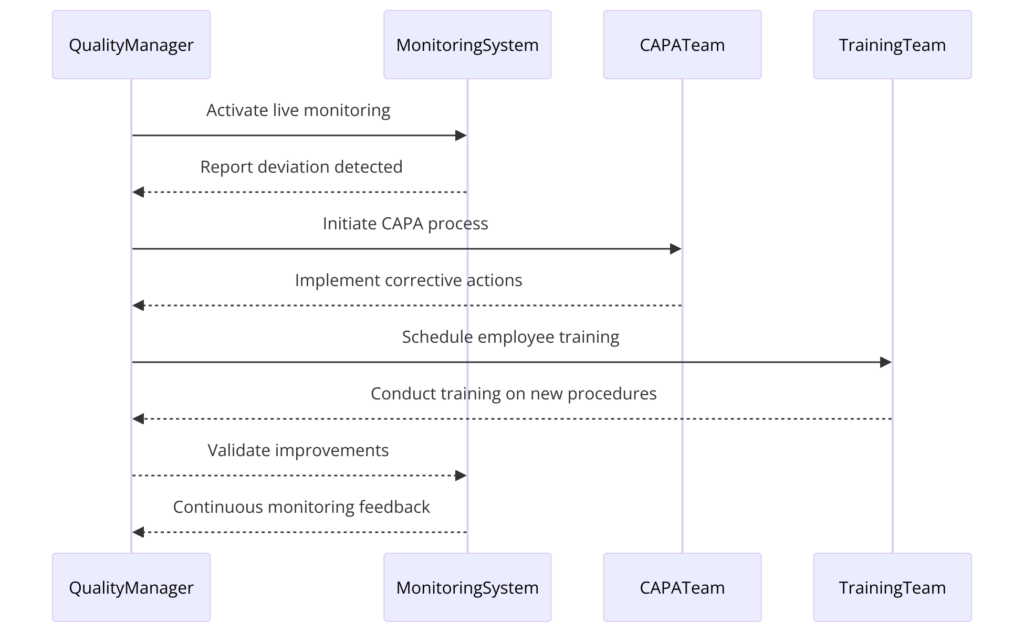
Having good quality products remains one of the hallmarks of pharmaceutical success. Quality failures, product recalls and regulatory violations not only damage a business reputation but also bankrupt it. For most CMOs and small pharma companies, outdated or disconnected quality control frameworks make it hard to observe deviations and take remedial action.
Product consistency and compliance demands the continuous upgrading or deployment of a strong QMS. Automated deviation management and CAPA processes allow businesses to respond to quality issues quickly and automatically. Combining live monitoring tools and standardized quality control processes helps improve supervision and minimize mistakes. A well-designed QMS helps you promote a culture of continuous learning to optimize long-term quality and compliance.
Strengthening Regulatory Compliance
The ever-changing, highly regulatory environment for pharma is an immense challenge for CMOs and small companies. When we do not comply with rigorous FDA, EMA, and global regulations, we risk harsh penalties, product recalls, and reputational harm.
In order to stay on top of regulatory compliance, proactive regulation compliance practices entail performing periodic gap analyses, storing up-to-date compliance matrixes, and creating strong documentation systems. The use of modern regulatory submission tools make filing and receiving regulatory filings less time-consuming and error-free, thus speeding up approvals. An in-depth understanding of regulatory needs and a robust compliance strategy ensures that firms will always be audit-ready and compliant.
Minimizing Production Downtime
Unexpected equipment breakdowns and production delays can disrupt manufacturing operations at an alarming rate, causing delays and costs. Almost all firms do not have formal maintenance plans and they are just doing reactive maintenance instead of proactive maintenance.
Preventive maintenance is the key to avoiding downtime and prolonging the life of production equipment. Automating maintenance schedules and sending out maintenance reminders ensures timely maintenance of machines. Automated monitoring services allow predictive maintenance so that businesses can detect issues and rectify them before they become catastrophic. Such predictive precautions protect production reliability and ensure optimal equipment performance.
Optimizing Supply Chain Resilience
Supply chain outages threaten pharmaceutical manufacturing. Depending on a small number of suppliers and mismanaging inventory can result in material shortages, production backlogs and higher prices. Resilience and adaptability are the key elements of an internationally regulated supply chain.
Creating a durable supply chain requires supplier diversification and strong inventory control. Analyzing supplier performance and creating secondary supply channels reduces supply disruptions. Implementing JIT inventory control ensures optimal stock levels, lower holding costs, and minimized waste. Providing better visibility and communication across supply chains increases overall operational flexibility.
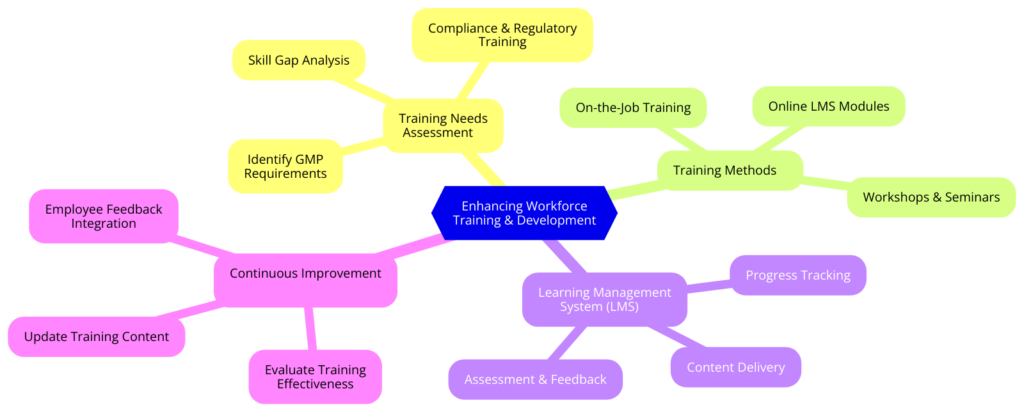
Enhancing Workforce Training and Development
A well-trained workforce is the foundation for both high production rates and compliance. Yet most CMOs and small pharma companies ignore the need to continuously train employees, leading to inefficiencies and regulatory risk.
Through designing robust training solutions for GMP, quality management, and regulatory compliance, employees are given the tools to perform at their best. Through learning management systems (LMS), trainings can be delivered and monitored consistently so that workers stay informed of current industry practices. Workshops and ongoing learning keep a culture of excellence and responsibility within the company strong.
Sustainable Growth Through Operational Excellence
Operational problems are inevitable in the fast-paced pharmaceutical space, but they’re not impossible. By leveraging a proactive, solutions-driven approach to process optimization, quality, capacity expansion, regulatory compliance, and workforce growth, CMOs and small pharmaceutical companies can turn these issues into growth opportunities.
By investing in cutting-edge technology, Lean practices and building a continuous improvement culture, companies are able to optimize, cut costs and deliver consistently high quality products. These bespoke services improve operational performance and set businesses up for success in an ever-increasingly competitive marketplace.
Setting a Foundation for Long-Term Success
Sustainable pharmaceutical industry growth requires more than short-term solutions. CMOs and smaller pharma organizations must work on building a foundation of operational excellence that can respond to changing industry needs. That platform is constructed by leveraging state of the art technology, data-driven decision making, and process improvement.
Firms should always be on the lookout for industry trends and regulatory changes. Through predictive analysis and real-time data, you can make more informed decisions and prevent the risks before they come crashing down. This preventative approach increases agility by allowing companies to respond quickly to market shifts and regulatory updates.
Moreover, developing a culture of continuous improvement promotes innovation at all levels. When people help solve the problem and improve processes, it means that they own and are responsible. Cross-functional collaboration between the production, quality, and supply chain teams ensures company goals are achieved and operational strategies implemented efficiently.
Combining technology investment, effective process control, and employee empowerment, CMOs and small pharmaceutical companies can not only overcome existing operational hurdles but be seen as leaders in efficiency, quality, and innovation.
Conclusion
Failures in CMOs and small pharma are operational issues that can undermine productivity, quality, and compliance if left unaddressed. But these challenges can be ripe for development if you approach them strategically. Organizations can monetize inefficiencies through process optimization, Lean Six Sigma, capacity development, quality improvement, regulatory readiness, and employee training.
With personalized solutions and an ongoing focus on innovation, CMOs and small pharma companies can achieve sustained growth, improve compliance, and improve product quality — all the while taking their place among the pharmaceutical pack.
Ready to optimize your operations and drive sustainable success? Contact us today to learn how our solutions can help your organization thrive.
